Introducing Wasabi by Dr Brian Oates
Introducing Wasabi (Wasabia japonica) for both the treatment and prevention
of COVID-19
Dr Brian Oates, President and Chief Science Officer
Wasabi Essentials Ltd
Vancouver, Canada
brian.oates@wasabia.com
604.351.0969 (available also through texting and WhatsApp)
Recent scientific evidence suggests that Wasabi (Wasabia japonica) helps to bothprevent and aid patients in recovery from COVID 19. Natural products from Wasabi accomplish this through restricting the virus’ ability to enter into human
cells and by blocking the advent of the cytokine storm syndrome (CSS) from appearing in lung tissue. This note presents information connecting the use of Wasabi to the treatment of COVID 19.
Traditionally grown in the river beds of Japan, Wasabi is a perennial plant that is a member of the Brassicaceae family. In many culinary contexts Wasabi is replaced by horseradish because of the cost and limited availability of the authentic
material. To distinguish between the two, the Japanese refer to it as hon wasabi or authentic wasabi. It is grown primarily for its rhizome, which, when finely grated, is used as a spice or condiment in high-end Japanese cuisine and is usually served as an integral part of raw fish dishes such as sushi or sashimi. Wasabi has many potent medicinal properties but has yet to be developed into a biomedical product because virtually all that is produced is sold into the culinary industry.
Wasabi is considered one of the hardest plants in the world to grow and it isbecause of this that the imitation Wasabi product made from horseradish wasdeveloped. Pacific Coast Wasabi has met this challenge and has generated methods
of growing Wasabi in greenhouses. Using these methods we have turned Wasabiinto a dependable crop that can be harvested year round. This means that the production of Wasabi is available on a continuous basis and that the development of biomedical products from this plant can take place.
Over the years, wasabi has slowly gained the interest of the medical field as a result of the suite of isothiocyanates (ITCs) that can be derived from the plant. Of these the most notable is 6-methylsulfonyl isothiocyanate (6MSITC) which is a very potent activator of the molecule NRF2. NRF2 is unique as it is a powerful stimulator of anti-oxidant production and is found in every cell in our bodies. The problem with NRF2 is that as we age it becomes less available to protect our cells from reactive oxygen spaces and other insults to our cells. Because of this, we become increasingly more prone to diseases (Dinkova-Kostova and Kazantsev 2017, Palacios et al 2018, Schmidlin et al 2019).
NRF2 was first discovered about twenty-five years ago and is only now gaining recognition as one of the most important molecules in the human body. In fact, in a 2015 editorial from the journal Arthritis Research & Therapy, Holger Jahr declared that: “NRF2 is probably the most important ubiquitously expressed little protein that you have never heard of”. As more research is being done surrounding NRF2, it is being found that it has been connected to a large number of human diseases (Dodson et al 2019).
There are many molecules that can activate NRF2. The most potent natural one is thought to be 6MSITC, which, as mentioned earlier is a natural product found almost exclusively in wasabi. In studies by Trio et al (2016, 2017), microarray technologies were used to show that the isothiocyanates of W. japonica activate NRF2. Korenori et al (2013) found similar links to activation using different techniques. In an earlier study Chen et al. (2010) showed that 6MSITC increased the expression of hundreds of genes and at the same time decreased the expression of hundreds of other genes. The authors did not examine the involvement of NRF2 in the regulation of these genes but it is clear that NRF2 was involved. Hou et al (2000) found similar findings with one gene and connected its transcription to the antioxidant/electrophile-responsive element (ARE) activation which in turn is induced by NRF2. Morimitsu et al (2002) found that 6MSITC activated NRF2 much more potently than sulforaphane, a more widely known isothiocyanate than 6MSITC. Mizuno et al (2011) showed that production of the cytoprotective glutathione is stimulated by NRF2 activation. Through the ability of 6MSITC to stimulate NRF2 Morroni et al (2014 and 2018) have shown that the molecule can protect against neurodegeneration and cognitive losses.
Several studies (Beeraka et al 2020, Cuadrado et al 2020, Lin and Yao 2020, McCord et al 2020, Mendonca and Soliman 2020, Zinovkine and Grebenchikov 2020, Bousquet et al 2021) are now suggesting that a powerful tool to be
considered in the fight against COVID is the activation of the body’s NRF2 pathway. There are multiple products that have been suggested to be capable of this but only a small amount of testing has been done to date. Through the activation of NRF2 there are two possible pathways that the genes upregulated by NRF2 can help fight against COVID 19:
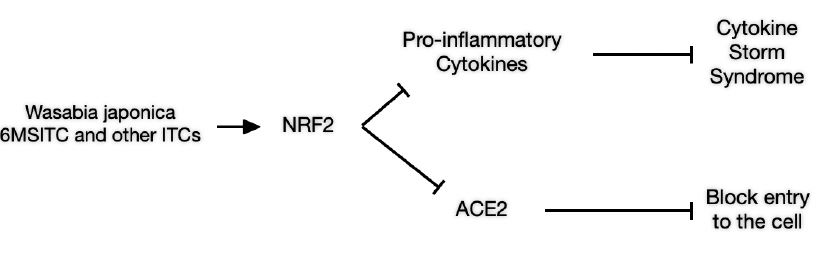
1. Through blocking of the production of pro-inflammatory cytokines that are involved in inflammatory syndrome known as Cytokine Storm Syndrome (CSS). CSS in the lungs leads to ICUs, respirators and often death.
2. NRF2 activation also blocks the production of angiotensin converting enzyme 2 (ACE2). The ACE2 molecule found on the surface of many cell types (oraland nasal mucosa, nasopharynx, lung, stomach, small intestine, colon, skin,
lymph nodes, thymus, bone marrow, spleen, liver, kidney, and brain [Hamming et al 2004]) presents the pathway through which the COVID virus enters the cell (Bian and Li 2021).
Fig. 1. Schematic of Wasabi effects on COVID-19 Patients based on the activation of NRF2.
What has become clear is that one of the body’s responses to COVID 19 can cause severe problems including viral pneumonia (Pfeifer and Hamer 2020, Brugge et al 2021). This response is the body’s over-production of inflammatory cytokines in response to the viral infection often the overproduction leads to the CSS. Cytokine storms are a common complication not only of COVID-19 and the seasonal flu but of other respiratory diseases caused by coronaviruses such as SARS and MERS. Cytokine storms are also suspected to be the main cause of death in the 1918 "Spanish Flu" pandemic and the 2005 outbreak of the avian H5N1 influenza virus, also known as “bird flu”.
Many studies (see Volmund et al 2017 and Hennig et al 2018 for reviews) have shown that cytokine production is regulated by NRF2. Kobayashi et al (2016) showed that NRF2 interferes with the up-regulation of pro-inflammatory cytokines including interleukin-6 and interleukin -1β (both IL-6 and IL—1β are prominent cytokines in CSS). Another example is the work of Ahmed et al (2017) where they reviewed the role of NRF2 in inflammation and showed that this molecule is involved in the down regulation of pro-inflammatory cytokines and also involved in the up-regulation of anti-inflammatory cytokines (eg. IL-10). A review by Zinovkin and Grebenchikov (2020) presented NRF2 as a prime candidate for reducing CSS and the authors presented numerous molecules that might act as activators of the NRF2. Timpani and Rybalka (2020) have studied the effects of dimethyl fumarate on calming CSS by elevating the NRF2 pathway and have shown that this pathway is effective. However, the authors, have also expressed some concerns about the molecule and have suggested that it only be used for severe cases.
activators of the NRF2. Timpani and Rybalka (2020) have studied the effects of dimethyl fumarate on calming CSS by elevating the NRF2 pathway and have shown that this pathway is effective. However, the authors, have also expressed
some concerns about the molecule and have suggested that it only be used for severe cases.
A final factor to take into consideration is the fact that COVID virus suppresses the expression of the NRF2 pathway which further exacerbates the situation (Olagnieret al 2020).
Observational Study
Supporting Literature and Online Sources
Ahmed SM, Luo L, Namani A, Wang XJ, Tang X. 2016. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis.1863(2):585-597. Epub 2016 Nov 4
Beeraka, N., Sadhu, S., Madhunapantula, S., Pragada, R., Svistunov, A.,Nikolenko, V., Mikhaleva, L., and Aliev, G. 2020. Strategies for Targeting SARS CoV-2: Small Molecule Inhibitors—The Current Status. Frontiers in
Immunology, 11:
Bian, J., and Li, Z. 2021. Angiotensin-converting enzyme 2 (ACE2):SARS-CoV-2
receptor and RAS modulator. Acta Pharmaceutica Sinica B, 11:1-12.
Bousquet, J., Moingc, V., Blaind, H., Czarlewskie, W., Zuberbiera,T., Torreh, R.,
Lozanoi, N., Reynesc, J., Bedbrookb, A., Cristolg, J., Cruzm, A., Fiocchin, A.,
Haahtelao, T., Iaccarinop, G., Klimekq, L., Kunar, P., Meléns, E., Mullolt, J.,
Samolinskiu, B., Valiulisv, A., and M. Antoi, J. 2020. Efficacy of broccoli and
glucoraphanin in COVID-19: From hypothesis to proof-of-concept with three
experimental clinical cases. World Allergy Organization Journal 14:1-16.
Brugge, S., Talman, S., Boonman-de Winter, L., Mol, M., Hoefman , E., Etten, R.,
Backer, I. 2020. Pulmonary function and health-related quality of life after
COVID-19 pneumonia. Respir. Med. 176:106272.
Chen, J., Uto, T., Tanigawa, S., Yamada-Kato, T., Fujii, M., and Hou, D. 2010.
Microarray-based determination of anti-inflammatory genes targeted by 6-
(methylsulfinyl)hexyl isothiocyanate in macrophages. Exp. Therapeut. Med. 1:
33-40.
Cuadrado, A., Pajares, M., Benito, C., Jiménez-Villegas, J., Escoll, M.,Angel J.
Yagüe, G,, Lastra, D., Manda, G., Rojo, A, and Dinkova-Kostova A.. 2020. Can
Activation of NRF2 Be a Strategy against COVID-19? Trends in
Pharmacological Sciences, September 41:b 598-609.
Dinkova-Kostova, A., and Kazantsev, A. 2017. Activation of Nrf2 signaling as a
common treatment of neurodegenerative diseases. Neurodegener. Dis. Manag.
7:97-100.
Dodson, M., Vega, M., Cholanians, A., Schmidlin, C., Chapman, E., and Zhang, D.
2019. Modulating NRF2 in disease: Timing is everything. Annu Rev Pharmacol
Toxicol59: 555–575.
Hamming, I., Timens, W., Bulthuis, M., Lely, A., Navis, G., and Goor, H. 2004.
Tissue distribution of ACE2 protein, the functional receptor for SARS
coronavirus. A first step in understanding SARS pathogenesis. J. Path.
203:631-7.
Hassan, S., Jawad, M., Ahjel, S., Singh, R., Singh, J., Awad, S., Hadi, N. 2020. The
Nrf2 Activator (DMF) and Covid-19: Is there a Possible Role? MED ARCH.
2020 APR; 74(2): 134-138
Hennig, P., Garstkiewicz, M., Grossi, S., Filippo, M., French, L. and-Beer, H.
2018. The Crosstalk between Nrf2 and Inflammasomes. Int J Mol Sci. 19: 562.
Hou D., Fukuda M., Fuji M., Fuke Y. 2000. Transcriptional regulation of
nicotinamide adenine dinucleotide phosphate: quinone oxidoreductase in murine
hepatoma cells by 6-(methylsufinyl)hexyl isothiocyanate, an active principle of
Wasabi (Eutrema wasabi Maxim). Cancer Letters, 161:195-200.
Hou, D., Korenori, Y., Tanigawa, S., Yamada-Kato, T., Nagai, M., He, X., He, J.
2011. Dynamics of Nrf2 and Keap1 in ARE-mediated NQO1 expression by wasabi 6-(methylsulfinyl)hexyl isothiocyanate. J. Agric. Food Chem. 59:
11975-82.
Jahr, H. 2015. HDACi and Nrf2: not from alpha to omega but from acetylation to
OA. Arthritis Research & Therapy, 17:381-2.
Kesic MJ, Simmons SO, Bauer R, Jaspers I. 2011. Nrf2 expression modifies
influenza A entry and replication in nasal epithelial cells. Free Radic BiolMed.
51:444–453.
Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H,
Tanaka N, Moriguchi T, Motohashi H, Nakayama K, Yamamoto M. 2016. Nrf2
suppresses macrophage inflammatory response by blocking proinflammatory
cytokine transcription. Nat. Commun. 23:11624.
Korenori, Y., Tanigawa, S., Kumamot, T., Gin, S., Daikoku, Y., Mivamori, K.,
Nagai, M., Hou, D. 2013. Modulation of Nrf2/Keap1 system by Wasabi 6-
methylthiohexyl isothiocyanate in ARE-mediated NQO1 expression. Mol. Nutr.
Food Res. EPub
Lin, C., and Yao, C. 2020. Potential Role of Nrf2 Activators with Dual Antiviral
and Anti-Inflammatory Properties in the Management of Viral Pneumonia.
Infection and Drug Resistance 2020:13 1735–1741.
McCord, J., Hybertson, B., Cota-Gomez, A., Geraci, K., and Gao, B. 2020. Nrf2
Activator PB125® as a Potential Therapeutic Agent against COVID-19.
Antioxidants, 2:518-33.
Mendonca, P., and Soliman, K. 2020. Flavonoids Activation of the Transcription
Factor Nrf2 as a Hypothesis Approach for the Prevention and Modulation of
SARS-CoV-2 Infection Severity. Antioxidants, 9, 659; doi:10.3390/
antiox9080659.
Mizuno, K., Kume, T., Muto, C., Takada-Takatori, Y., Izumi, Y., Sugimoto, H., and
Akaike, A. 2011. Glutathione biosynthesis via activation of the nuclear factor
E2-related factor 2 (Nrf2) - antioxidant-response element (ARE) pathway is
essential for neuroprotective effects of sulforaphane and 6-(methylsulfinyl)
hexyl isothiocyanate. J. Pharmacol. Sci. 115(3):320-8.
Morimitsu, Y., Nakamura, Y., Hayashi, K., Fuji, K., Kumagai, H., Nakamura, T.,
Osawa, Y., Horio, T., Itoh, F., Lida, K., Yamamoto, K., and Uchida, M. 2002. A
sulforaphane analogue that potently activates the Nrf2-dependent detoxification
pathway. J. Biol. Chem. 277: 3456-63.
Morroni, F., Sita, G., Tarozzi, A., Cantelli-Forti, G., and Hrelia, P. 2014.
Neuroprotection by 6-(methylsulfinyl)hexyl isothiocyanate in a 6-
hydroxydopamine mouse model of Parkinson's disease. Brain Research,
Morroni, E., Sita, G., Graziosi, A, Turrini, E., Fimognari, C., Tarozzi, A., and
Hrelia, P. 2018. Protective Effects of 6-(Methylsulfinyl)hexyl Isothiocyanate on
Aβ1-42-Induced Cognitive Deficit, Oxidative Stress, Inflammation, and
Apoptosis in Mice. Int. J. Mol. Sci. 19:2083 (19 pages).
Olagnier, D., Farahani, E., Thyrsted, J., Blay-Cadanet, J., Herengt, A., Idorn, M.,
Hait, A., Hernaez, B., Knudsen, A., Iversen, M., Schilling, M., Jørgensen, S.,
Thomsen, M., Reinert, L., Lappe, M., Hoang, H., Gilchrist, V., Hansen, A.,
Ottosen, R., Nielsen, C., Charlotte Møller, C., Horst, D., Peri, S., Balachandran,
S., Huang, J., Jakobsen, M., Svenningsen, E., Poulsen, T., Bartsch, L., Thielke,
A., Luo, Y., Alain, T., Rehwinkel, J., Alcamí, A., Hiscott, J., Mogensen, T.,
Paludan. S., and Holm, C.2020. SARS-CoV2-mediated suppression of NRF2-
signaling reveals potent antiviral and antiinflammatory activity of 4-octylitaconate
and dimethyl fumarate. NATURE COMMUNICATIONS | (2020)
11:4938.
Palacios, S., Ostolga-Chavarría, M., Zazueta, C., and Königsberg, M. 2018. Nrf2:
Molecular and epigenetic regulation during aging. Ageing Research Reviews,
47:31-40.
Pfeifer, M, and Hamer O. 2020. COVID-19 pneumonia. Internist (Berl.) Jul 29 : 1–
10.
Schmidlin, C., Dodson, M., Madhavan, L., and Zhang, D. 2019. Redox regulation
by NRF2 in aging and disease. Free Radic. Biol. Med. 134:702-707.
Timpani, C., and Rybalka, E. 2020. Calming the (Cytokine) Storm: Dimethyl
Fumarate as a Therapeutic Candidate for COVID-19. Pharmaceuticals 2021, 14,
15. https://dx.doi.org/10.3390/ph14010015
Trio, P., Fujisaki, S., Tanigawa, S., Hisanaga, A., Sakao, K. and De-Xing. 2016.
DNA microarray highlights Nrf2-mediated neuron protection targeted by
Wasabi-derived isothiocyanates in IMR-3q2 cells. Gene Regul Syst. Bio.
10:73-83.
Trio, P., Kawahara, A., Tanigawa, S., Sakao, K., and Hou, D. 2017. DNA
microarray profiling highlights Nrf2-mediated chemoprevention targeted by
wasabi-derived isothiocyanates in HepG2 cells. Nutr. Can. 69:105:116.
Vomund, S. Schäfer,A., Parnham, M. Brüne, B., and Knethen, A. 2017. Nrf2, the
Master Regulator of Anti-Oxidative Responses. Int J Mol Sci. 18: 2772.
Zinovkin, R., and Grebenchikov, O. 2020. Transcription Factor Nrf2 as a Potential
Therapeutic Target for Prevention of Cytokine Storm in COVID19 Patients.
Biochemistry (Moscow), 85:978-83.aging
Some online references:
https://www.newscientist.com/term/cytokine-storm/
https://www.france24.com/en/20200323-researchers-study-drug-to-reducecovid-
19-complications
https://www.vox.com/2020/3/12/21176783/coronavirus-covid-19-deaths-chinatreatment-
cytokine-storm-syndrome